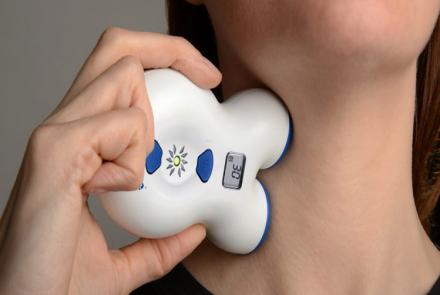
ElectroCore, LLC (electroCore), a commercial-stage bioelectronic medicine company received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for an expanded label for gammaCore® (nVNS) as an acute treatment of pain associated with migraine in adult patients.
gammaCore therapy is a proprietary, non-invasive neuromodulation treatment delivered by a hand-held unit that stimulates the vagus nerve through the skin. This follows the initial FDA clearnance receieved by gammaCore for…
Migraine
- Worldwide, medical hypnosis is being increasingly accepted to ease acute and chronic pain arising from burns, cancer, and rheumatoid arthritis and reduction of anxiety associated with surgery. What is hypnosis? Hypnosis is a state of increased suggestibility with constriction of peripheral awareness and increased focal concentration on task at hand. Heightened suggestibility is an essential characteristic in hypnosis. Hypnosis is like a meditative technique that encourages inner search and the…